
正文共:17544 字,28 图
预计阅读时间:45 分钟
1981年初夏,在某一期的《科学》期刊上登载了一则极为简单的招聘广告,一家科技初创公司要招聘一名新药研究员。可能这家公司想要省点钱,因此这则广告的字数有限,既没公司名称,也没职位介绍,并没有引起多少读者的关注。不过,还是有位四十岁的科学家给该公司递了履历,这点有限的广告费没有白花。这位求职者似乎在其它地方已经走投无路了,他一拿到聘书,就将全部家当卷入了几只行李箱,带着太太从美国东岸举家搬到了这家公司的所在地加州千橡市。在上世纪80年代初,这家公司微小得像一颗尘沙,如果经历的风雨稍大,它都会被冲刷得不知去向。这位科学家在事业上也陷入了困境,对工作早没有了挑挑拣拣的“雅好”,只要能够有一份专业对口的工作得以养家糊口,他都能接受。就这样,当时都看似“不幸”的一家初创公司和一位科学家,碰撞出了一点“小确幸”。
只有时间才能告诉我们这一次巧合的真正的价值和意义,三十九年之后回头再望,正是这家公司的无奈和这位科学家的落魄,让他们走在了一起,最终成就了一家全球前十大的生物制药公司Amgen(安进)。2020年2月,安进的股票市值1200亿美元;2019年全年,安进的年销售额为234亿美元。这,就是当年那一点点“小确幸”最后爆发出的“大价值”。
这一位科学家出生在中国台湾基隆,名叫林福坤。青春年少,他离乡别井到美国留学时,也曾经意气风发,怀抱着男子汉志在四方的壮志,打算在学术上成就一番事业。30岁时,他在美国伊利诺大学获得了植物学博士学位。但是,毕业之后的前十年,他一直都行走在人生的低谷中,似乎刚有一扇门为他开启,但很快又被无情地关上了。为了生计,他不断往返于太平洋两岸,换了五份工作,后来在南卡罗来纳医科大学做一些研究。这样的动荡流离,让他早已偏离了本来的专业,进入了基因工程研究。到了不惑之年40岁,林福坤依然不知道自己的天命何在,只知道生存比天大。这个其他科学家丝毫看不上的工作机会,也会吸引他毅然决然地投奔而去。最起码,它可解决一家人的生计啊!没有想到的是,如此寒微的一个起点,却让林福坤取得了足以载入人类医药历史的丰功伟绩。


这些钱在当年堪为巨资。最初,他们试图从页岩中提取石油的微生物;后来,他们又努力让养殖场的鸡长得更快;再后来,他们又开始制造各种特殊化学品,比如克隆荧光素基因等等。可惜,白花花的银子都像是丢进了海里,败光了几千万美元后,他们还是一事无成。不幸中的万幸是,安进的第七号科研人员在1981年正式报到了,他就是后来挽狂澜于既倒、扶大厦于将倾的林福坤。
基因新秀 逆势而生
具体工作中,林褔坤选中了促红素基因EPO为自己的研究方向。EPO是由肾赃分泌的一种激素蛋白,能刺激骨髓产生红血球。肾功能衰竭的人,EPO的分泌会随之而减少,导致严重贫血。在获取EPO方面,林福坤不是开创者。1970年代时,芝加哥大学的Goldwasser教授已从再生障碍性贫血患者的尿液中分离纯化出EPO,但含量极微,收集2500公升尿液后才能提取出区区几毫克的EPO,所以这种办法并无实用价值。当时人们对基因重组DNA所知甚少,基因测序和克隆所需要的技术方法也非常原始。人类EPO基因具有庞大的氨基酸序列,那个年代,人们想要寻找和分离出EPO基因,如同大海捞针般困难。当时安进的科研条件如此捉襟见肘,要钱缺钱又要人缺人,林福坤可以突围而出的可能性似乎更加渺茫。
然而,运气一旦来了,任何困难面前都是逢山开路、遇难成祥。此前一辈子几乎都一事无成的林福坤,生命竟然被EPO点亮了。1983年底,他与唯一的一位助手——台大学妹林启辉从150万个基因片段中成功分离出EPO序列。这个促红素EPO,理论上可以治疗多种原因引起的贫血,具有巨大的药用潜力。

安进拿到了强生的续命款后,EPO的临床试验才得以继续。与此同时,安进已有多个产品开始了人体试验,包括乙肝疫苗、三种细胞因子和两种干扰素。很快,EPO的巨大潜力就在三期临床中快速地被展露了出来。1987年,安进以Epogen为药名向FDA提交了上市申请。该药于1989年获批,成为了安进第一个成功上市的药品。1990年,它的销售额飙升到1.4亿美元,人们称其为“红药”,被《财富》杂志评为年度最佳药品。此外,安进公司还有另一个由拉里·索萨博士(Larry Souza)领导的研究小组,在1985年成功地克隆出了粒细胞集落刺激因子 (G-CSF) ,主要用于治疗癌症放疗或化疗后引起的白细胞减少症。1991年,该药被命名为 Neupogen,也被FDA批准上市, 第一年就创造了2亿多美元的销售,人们称其为“白药”,同样也被《财富》杂志评为年度最佳产品。

更上层楼 管线多发
另外,AMG-510也表现出良好的安全性和耐受性,治疗相关的不良事件(TRAEs)发生率仅为35%, 其中大多为1、2级,而且没有4级或致命的AE。最常见的AE为腹泻、恶心和一例贫血。因为该药效果良好,FDA已授予它未来快速通道审批的认定。AMG -510单药治疗NSCLC的关键性二期临床现已启动,另加一个与PD-1 (Keytruda)的组合疗法二期临床。安进表示,还将继续探索AMG-510与其它疗法可能取得协同效果的临床试验。2020年1月,安进宣布与Guardant Health和Qiagen 公司达成合作协议,共同开发基于血液和组织的KRAS伴随诊断产品(Companion Diagnostic), 以便进一步加强AMG-510的成功机会。
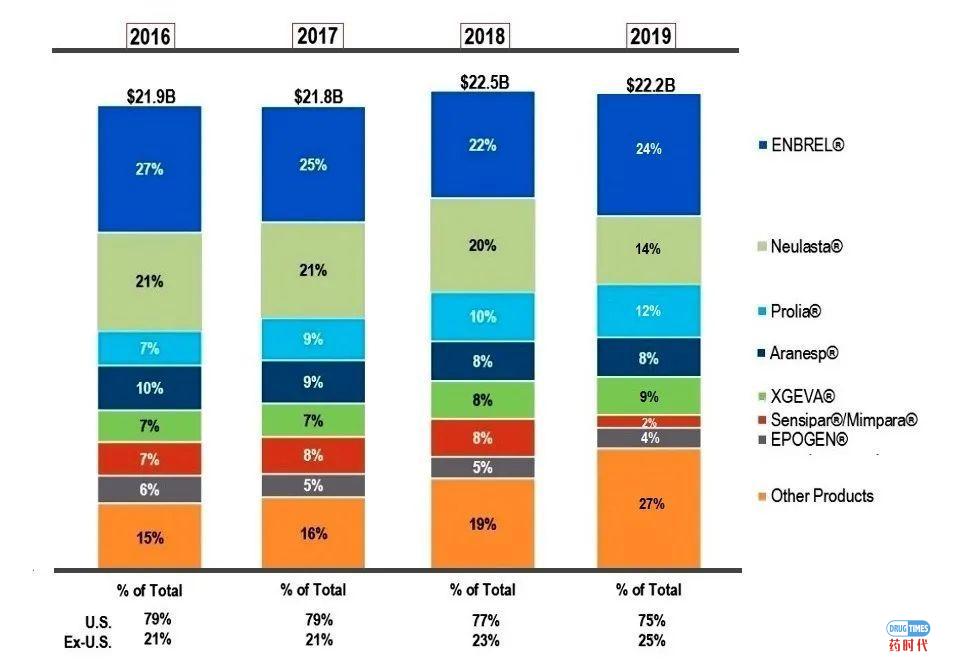
近年来,安进投入了大量资金用以提高公司内部的科研能力,同时加强与各个科研院校的紧密合作。2001年,安进在麻省剑桥市修建了最新的科创中心;还和MIT等高校合作设立科研中心。除了引进人才、招聘顶尖科学家、积极对外并购、补充研发管线、以及收购重磅大药外,安进还有哪些其他公司并不常见的绝杀秘籍呢?第一大奇招就是:霸气的专利诉讼策略。
与其它大型药企的画风不同,紧密陪伴着安进科研之路的还有一条法律诉讼之路,或者说,安进比其它药企更加懂得如何积极地利用法律武器,来保护公司的市场份额:
然而,安进与强生的官司并未就此结束。1991年,强生又以安进违反1985年销售协议为名,再次把安进告上法院,安进被判赔偿1.6亿美元。对于刚刚缓过元气的安进而言,这笔索赔相当于当时的全部家当,安进不得不做好破产的心理准备,同时抱着背水一战的心态进行了上诉。戏剧性的是,该案的局势在次年发生了反转,安进以强生未履行该合同规定而开发乙肝疫苗和IL-2,并且未按合同支付授权费为由,要求强生赔偿9000万美元。两家公司的恩怨此后还在继续,1998年,法院判决安进因长期违反合同销售Epogen而需向强生支付2亿美元,不过也同时要求强生赔偿安进在长期争议中所损失的1亿美元。
由于有了与安进PCSK9连年诉讼的教训,再生元害怕自己的新药Dupixent(IL-4R 抗体)也会受到同样命运,所以决定先下手为强;2017年3月,再生元申诉地方法院,要求法官理清其Dupixent并未侵犯安进专利。2017年4月,安进正式起诉再生元侵犯其IL-4R专利。安进认为,再生元在其Dupixent的专利中表明,在开发过程中,它依赖于安进的mAb 12B5作为对照组抗体,并开展了结合亲和力测试, 这从而证实了再生元对安进的专利非常了解,因此是故意侵权。与PCSK9诉讼不同,安进并未寻求对再生元Dupixent发起禁令,因为安进目前没有销售竞争产品 (AMG-317在2009年时的哮喘二期临床失败)。相反,安进只要求再生元为侵犯其专利而赔偿特许权使用费(Royalty)。不过,2019年2月,美国专利局宣布安进在IL-4R的17项专利权利要求(Patent Claims)均无效,因为数据并没创新而且非常“明显”(Obvious) 。欧洲专利局随后也撤销了同一安进专利权利要求,原因是安进在专利信息上披露不足。对于这一判决,善于打官司的安进表示,还将寻求上诉。
讽刺的是,安进自己也在做生物类似药。换到了这个角度,它同样也善于运用法律武器来保护自己,其法律团队积极并频繁地向其他原研药厂发起专利挑战。2019年8月底,安进挑战Alexion公司天价抗体药Soliris (C5单抗)的专利,为其Eculizumab上市铺路。因为安进在专利挑战上的鼎鼎霸名,此举一出,Alexion公司便闻风丧胆,股价一路狂跌。分析师估计,安进开发的生物类似药Rituximab, Infliximab, 和Eculizumab 在2023年的销售额将为28亿美元。
有人戏称,安进其实是一家以医药为主的“律师事务所”,各种授权费和赔偿金才是其真正收入来源。弱小的时候,生存总是不易,成立之初的安进为了一千万美元的“生存金”而被迫向强生屈膝。这一千万美元救了当时的安进,却为其带来了一系列的法律麻烦。但就是在与强生的斗争中,这一千万美元又变相地锻炼了安进的筋骨,不仅建立了它的EPO帝国和与之相关的千亿美元收入,还将安进培养得比任何其它一家公司更懂得利用法律武器来助攻战略版图。
至于林福坤,他为安进打下江山的汗马功劳绝对值得写入制药业的历史。出人意料的是,他于1998年57岁时就提早退休。作为安进的第7号员工,当安进从一家小型的初创企业成长为一万多名员工的大药企后,他在公司的地位自然就无法像从前一样举足轻重了。随着公司的成长,原来自由灵活的研发氛围也逐渐消失了,让他心生了卸甲归田之念。退休后的林福坤,与朋友合作在台湾创立了一家名为”药华医药“的生物科技公司。不过,他再也没能重写在EPO上铸就的辉煌过去。此时此刻,在加州的田园中,功已成身已退的林福坤正和太太过着采菊东篱下,悠然见南山的写意生活。他的人生没有任何遗憾,这本来就是最为朴素和真实的幸福。
在您的朋友圈里,有没有曾为大药企做出重大贡献的朋友呢?他们公司是如何对待他们的呢?他们有没有获得巨额奖励,并被晋升为公司高管?或者,他们现在还只是继续在实验室中默默耕耘的板凳科学家?如果您是林福坤,您也会选择离开大药企后创业吗?我们期望听取您的心得,并与其他读者分享。
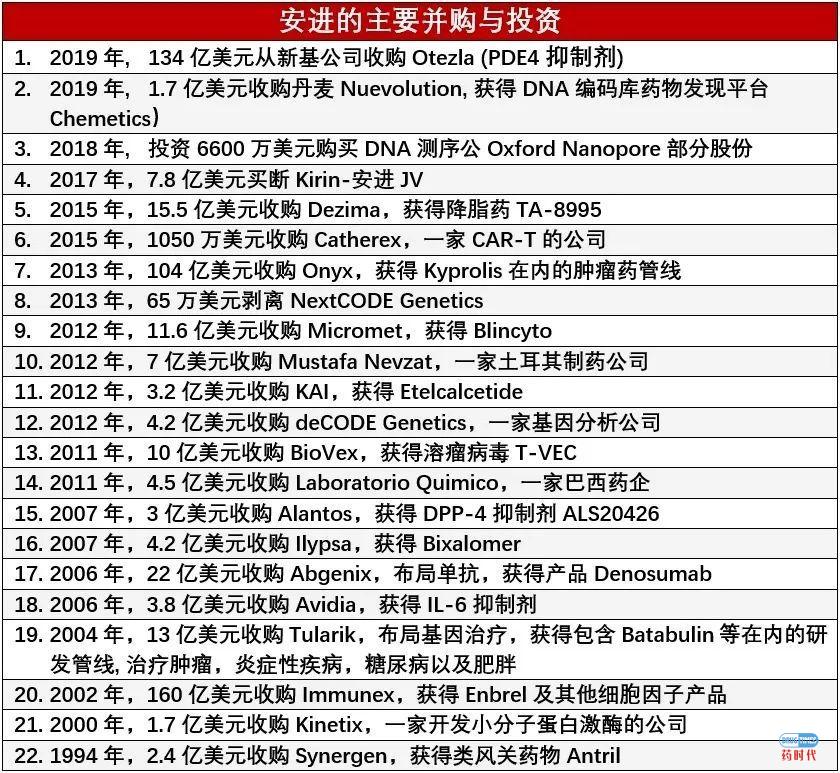
Detailed Description of Phase 1 Assets of Amgen
AMG 119 is a DLL3 CAR-T cellular therapy. It is being investigated in a Phase 1 study for small-cell lung cancer. AMG 160 is a half-life extended anti-PSMA x anti-CD3 BiTE. It is being investigated in a Phase 1 study for prostate cancer. AMG 171 is a GDF15-Fc fusion protein being investigated in a Phase 1 study as an anti-obesity treatment. AMG 176 is an intravenous small molecule inhibitor of MCL-1. It is being investigated in a Phase 1 study for hematologic malignancies. AMG 199 is a MUC17 targeting half-life extended BiTE. It is being investigated in a Phase 1 study for metastatic gastric and gastroesophageal junction cancer. AMG 212 is an anti-PSMA x anti-CD3 BiTE. It is being investigated in a Phase 1 study for prostate cancer. AMG 330 is an anti-CD33 x anti-CD3 BiTE. It is being investigated in a Phase 1 study for acute myeloid leukemia. AMG 397 is an oral small molecule inhibitor of MCL-1. It is being investigated in a Phase 1 study for hematologic malignancies. AMG 404 is a human anti-PD-1 monoclonal antibody being investigated as a treatment for patients with solid tumors. It is being developed in a Phase 1 study for use in combination with other Amgen oncology portfolio molecules. AMG 420 is an anti-BCMA x anti-CD3 BiTE. It is being investigated in a Phase 1 study for multiple myeloma. AMG 424 is a bispecific anti-CD38 x anti-CD3 T cell-recruiting antibody (XmAb). It is being investigated in a Phase 1 study for multiple myeloma. AMG 427 is a half-life extended anti-FLT3 x anti-CD3 BiTE. It is being investigated in a Phase 1 study for acute myeloid leukemia. AMG 430 is an anti-Jagged-1 monoclonal antibody being investigated in a Phase 1 study for respiratory diseases. AMG 506 is a multi-specific FAP x 4-1BB-targeting DARPin. It is a biologic under investigation as a treatment for patients with solid tumors. It is being developed for use in combination with other Amgen oncology portfolio molecules. AMG 506 (also known as MP0310) is being developed in collaboration with Molecular Partners AG. DARPin is a registered trademark owned by Molecular Partners AG. AMG 562 is a half-life extended anti-CD19 x anti-CD3 BiTE. It is being investigated in a Phase 1 study for non-Hodgkin’s lymphoma. Efavaleukin alfa is an IL-2 mutein Fc fusion protein. It is being investigated in a Phase 1 study for rheumatoid arthritis, systemic lupus erythematosus and graft versus host disease. AMG 594 is a cardiac troponin activator. It is being investigated in a Phase 1 study for heart failure. AMG 594 was discovered under a joint research program conducted between Amgen and Cytokinetics. AMG 596 is an anti-EGFRvIII x anti-CD3 BiTE. It is being investigated in a Phase 1 study for glioblastoma. AMG 673 is a half-life extended anti-CD33 x anti-CD3 BiTE antibody construct. It is being investigated in a Phase 1 study for acute myeloid leukemia. AMG 701 is a half-life extended anti-BCMA x anti-CD3 BiTE. It is being investigated in a Phase 1 study for multiple myeloma. AMG 757 is a half-life extended anti-DLL3 x anti-CD3 BiTE. It is being investigated in a Phase 1 study for small-cell lung cancer. AMG 890 is a small interfering RNA (siRNA) that targets lipoprotein(a), also known as Lp(a). It is being investigated in a Phase 1 study for cardiovascular disease.
Detailed Description of Phase 2 and 3 Assets of Amgen
-
AMG 510 is a KRASG12C small molecule inhibitor. It is being investigated in a Phase 2 study for non-small cell lung and colorectal cancers. -
Rozibafusp alfa is a bispecific antibody-peptide conjugate that targets BAFF and ICOS ligand. It is being investigated in a Phase 2 study for systemic lupus erythematosus. -
AMG 714 is a human monoclonal antibody that binds to IL-15. It is being investigated in a Phase 2 study for celiac disease. It is developed in collaboration with Provention Bio, Inc., where it is identified as PRV-015. -
Tezepelumab is a human monoclonal antibody that inhibits the action of thymic stromal lymphopoietin (TSLP). It is being investigated in a Phase 3 study for severe asthma. In September 2018, FDA granted Breakthrough Therapy Designation for tezepelumab in patients with severe asthma without an eosinophilic phenotype. Tezepelumab is being developed in collaboration with AstraZeneca. It is also investigated in a Phase 2 study for atopic dermatitis as well as in another Phase 2 study for COPD.
-
Aimovig inhibits the calcitonin gene-related peptide receptor (CGRP-R). It is being investigated in a Phase 3 study for prevention of chronic and episodic migraine in pediatric patients.
-
BLINCYTO is an anti-CD 19 x anti-CD3 BiTE. It is being investigated in a Phase 3 study for acute lymphoblastic leukemia (ALL) in pediatric patients at first relapse.
-
EVENITY is a humanized monoclonal antibody that inhibits the action of sclerostin. It is being investigated in a Phase 3 study for male osteoporosis.
-
IMLYGIC is an oncolytic immunotherapy derived from herpes simplex virus type 1 (HSV-1). It is being investigated in a Phase 3 study as a combination treatment with KEYTRUDA in patients with mid- to late-stage metastatic melanoma.
-
KYPROLIS is a small molecule proteasome inhibitor. It is being investigated in the Phase 3 ARROW-2 study for weekly dosing in combination with lenalidomide and dexamethasone for relapsed multiple myeloma. It is also being investigated in the Phase 3 CANDOR study in combination with dexamethasone and DARZALEX compared to KYPROLIS and dexamethasone alone.
-
Nplate is a thrombopoietin (TPO) receptor agonist. It is being investigated in a Phase 3 study for early chemotherapy-induced thrombocytopenia.
-
Omecamtiv mecarbil is a small molecule selective cardiac myosin activator, also called a myotrope, which directly targets the contractile mechanisms of the heart. It is being investigated in Phase 3 studies for the potential treatment of heart failure with reduced ejection fraction. Omecamtiv mecarbil is being developed under a collaboration between Amgen and Cytokinetics, with funding and strategic support from Servier.
-
Otezla is a small molecule that inhibits phosphodiesterase 4 (PDE4). It is being investigated in several Phase 3 study for oral ulcers associated with Behcet’s disease, pediatric plaque psoriasis, and severe genital psoriasis, respectively.
-
Parsabiv is a peptide that binds to the calcium-sensing receptor. It is being investigated in a Phase 3 study in pediatric patients with secondary hyperparathyroidism and chronic kidney disease receiving hemodialysis.
-
Prolia is a monoclonal antibody that inhibits RANK ligand. It is being investigated in a Phase 3 study in pediatric patients with glucocorticoid-induced osteoporosis.
-
Repatha is a human monoclonal antibody that inhibits proprotein convertase PCSK9. It is being investigated in the Phase 3 VESALIUS-CV study in high-risk cardiovascular disease patients without prior heart attack or stroke as well as in another Phase 3 study in pediatric patients with familial hypercholesterolemia.
Detailed Description of BioSimilar Phase 3 Assets of Amgen
-
ABP 798, a biosimilar candidate to rituximab (Rituxan./Mabthera.), is an anti-CD20 monoclonal antibody. It is being investigated in a Phase 3 study for non-Hodgkin’s lymphoma and RA, respectively. The reference product primary indications are non-Hodgkin’s lymphoma, chronic lymphocytic leukemia and rheumatoid arthritis. Amgen is developing ABP 798 in collaboration with Allergan.
-
ABP 959, a biosimilar candidate to eculizumab (Soliris), is a monoclonal antibody that specifically binds to the complement protein C5. It is being investigated in a Phase 3 study for paroxysmal nocturnal hemoglobinuria. The reference product primary indications are PNH and atypical hemolytic uremic syndrome.
参考资料
1.http://investors.amgen.com/news-releases/news-release-details/amgen-files-lawsuit-against-sanofi-and-regeneron-patent
2.http://investors.amgen.com/news-releases/news-release-details/amgen-resolves-epo-patent-dispute-roche
3.https://boingboing.net/2010/07/21/the-2-billion-error.html
4.https://en.wikipedia.org/wiki/Amgen
5.https://en.wikipedia.org/wiki/Fu-Kuen_Lin
6.https://www.amgen.com/
7.https://www.amgenbiotech.com/manufacturing-innovation.html
8.https://www.amgenpipeline.com/
9.https://www.bloomberg.com/profile/company/AMGN:US
10.https://www.fiercepharma.com/pharma/not-quite-a-year-into-launch-amgen-wants-to-can-its-aimovig-collaboration-novartis-lawsuit
11.https://www.forbes.com/companies/amgen/#3c82d5e56ae3
12.https://www.marketwatch.com/investing/stock/amgn
13.https://www.nytimes.com/2002/10/19/business/johnson-johnson-to-pay-150-million-in-amgen-suit.html
14.https://www.policymed.com/2013/02/amgen-settlement-and-corporate-integrity-agreement.html
15.https://www.wsj.com/articles/amgen-tasks-its-labs-with-finding-new-blockbusters-11577638800
版权声明:文中图片取自网络,根据CCO协议使用,版权归拥有者。任何问题,请与我们联系。衷心感谢!

推荐阅读
2020-02-27

2020-02-06

2020-01-08

2019-12-29

2020-01-03

2019-12-19

2019-12-13

2019-10-13

2019-11-27

2019-11-20

2019-11-13

2019-10-28

2019-10-22



发布者:药时代,转载请首先联系contact@drugtimes.cn获得授权

 为好文打赏 支持药时代 共创新未来!
为好文打赏 支持药时代 共创新未来! 
